The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
ClosePatient Story
- Endocrine & Metabolic
- Immunology
- Neuroscience
- Cell & Gene Therapy
Jason lived with the disease of obesity, until a letter changed his life.
He felt alone. Hopeless. Like nothing could change.
At 32, Jason had been bullied for years about his obesity, a disease that affects 1 in 8 people around the world.
Jason decided it was time to make a change.
In a desperate attempt to show he was going down the same path as his late father, Jason’s mother bought him a burial plot.
When the letter arrived notifying him, it shook him.
Over the next few years, he dieted, exercised, and underwent bariatric surgery twice. He started GLP-1 medications — and the weight came off.
Today he continues to advocate for legislation, educates the public, and encourages clinical trial participation.
He also collaborates with Parexel so we can better understand how to meet the needs of patients with obesity.
Jason became a patient advocate. He even got a bill passed in New Jersey recognizing Obesity Care Week in October.
Lives can change when you design endocrine & metabolic trials with speed and precision.
- Find and engage the patients you need
- Pinpoint the perfect sites for your trial
- Access the right experts, focused on the right indications
- Collect and utilize reliable, high-quality data
- Satisfy global regulations to get your treatment to market safely and quickly
What we do, we do

Our Experts
Our obesity and metabolic specialists collaborate to get your treatment to patients faster.

Our Experts
Shipra Patel, M.D.
Global Therapeutic Area Section Head, Endocrinology & Metabolism
Dr. Shipra Patel partners with sponsors to design and deliver innovative and efficient solutions for GLP-1 and metabolic programs across all phases of development. A board-certified pediatric endocrinologist with more than 20 years of clinical experience, she leads Parexel’s Endocrinology & Metabolism team, providing strategic oversight and scientific guidance to advance representative enrollment, patient retention, and accelerated delivery of new therapies for patients worldwide.
“In obesity trials, we ‘recruit to retain’ with clear expectations, honest prescreening, and engaged sites so participants feel supported and stay in the study.”
Our extensive project experience ensures you get the expertise you need across every indication in endocrine & metabolism, gastrointestinal, and cardiovascular clinical research.
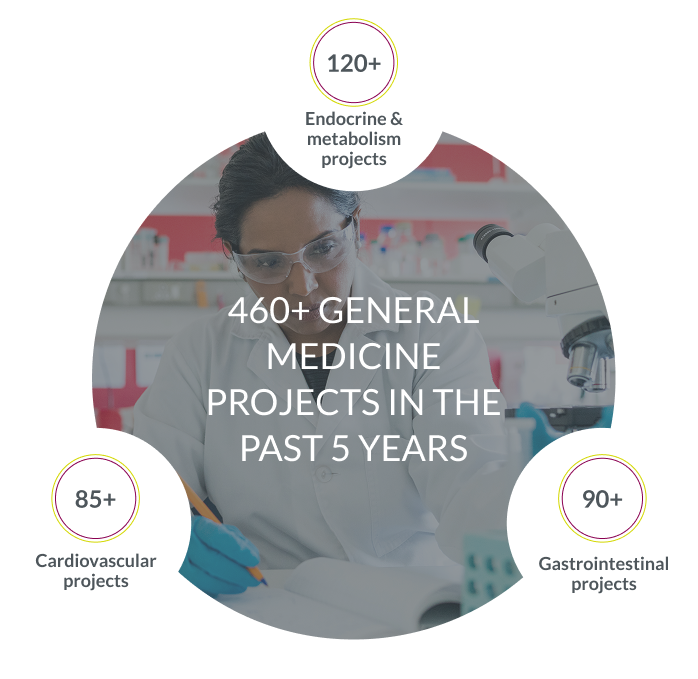
Our global experience with general medicine trials, including our 1,390 alliance sites dedicated to endocrine & metabolic studies, enables faster site activation and accelerates study start-up across key regions.
- North America
- South America
- Europe
- APAC
- Middle East & Africa

Our highly tailored recruitment and retention strategies enable access to cardio-metabolic patient populations across obesity, diabetes, and cardiovascular studies worldwide.
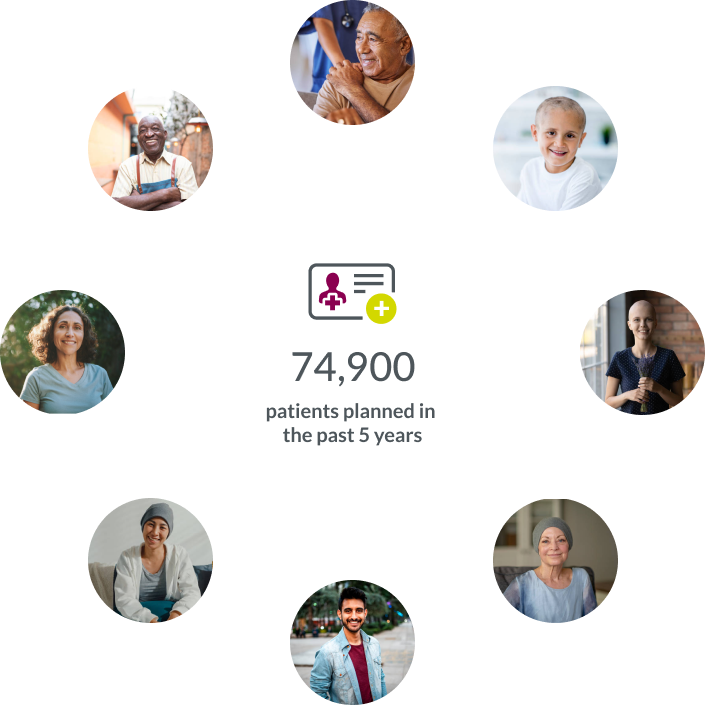
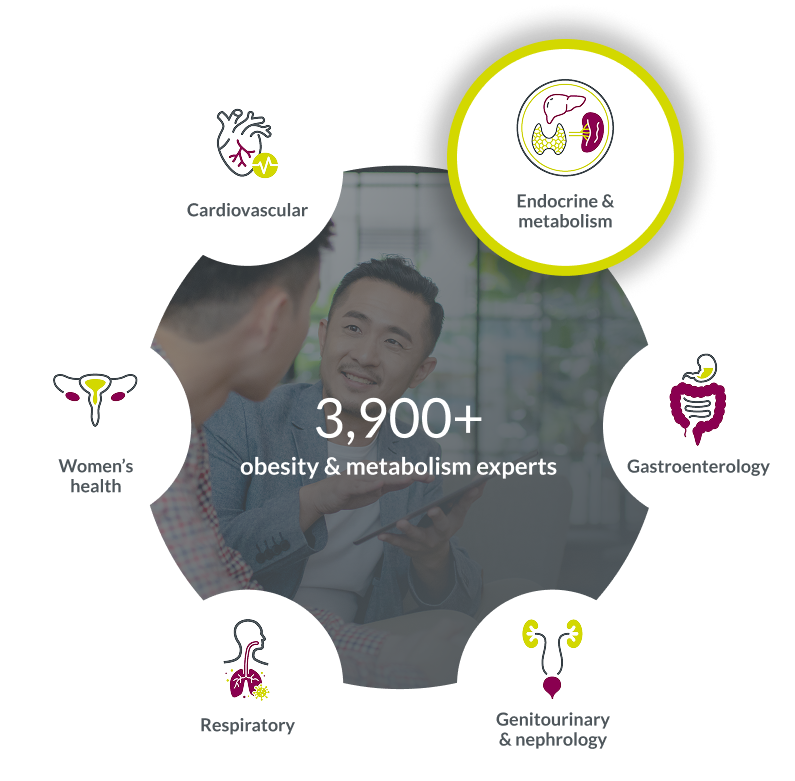
Our general medicine team is 8,500+ strong, including 3,900+ experts supporting obesity and metabolism studies.
And our regulatory team includes former FDA, EMA, and HPRA regulators, with experience across various treatments, including GLP-1 medications.

What can we do to help you change patient lives?
Endo-metabolic capabilities All therapeutic areas General medicine careers
Discover other patient stories
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
CloseWe focus on patients, because they inspire us to deliver better clinical trials, faster than ever. So we can make a difference for more patients like Jason.
Who we are,
Parexel is proudly among the world’s largest
clinical research organizations
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 24,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind, to make clinical research a care option for anyone, anywhere.



