The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
ClosePatient Story
- Cell & Gene Therapy
- Immunology
- Neuroscience
- Endocrine & Metabolic
One night, while watching TV with her husband, Robyn felt a lump in her neck.
She had large B-cell lymphoma — an aggressive cancer diagnosed in 150,000 patients each year globally.
As a doctor, she knew the risk. She got a CAT scan, looked at the images, and her world stopped.
Four years later the lymphoma returned. Robyn’s care team prescribed a further six cycles of a brutal chemotherapy, followed by an autologous stem cell transplant (ASCT) and external beam radiation. Robyn’s ASCT treatment was further complicated by septic shock requiring a stay in intensive care.
Robyn entered remission again, but it was short-lived, with the lymphoma returning nine months later. Treatment options for Robyn were now bleak and limited.
Robyn immediately sought treatment. With her husband by her side, she underwent many rounds of chemotherapy and went into remission.
Feeling defeated, she found a clinical trial for CAR T-cell therapy, a new treatment that reengineers white blood cells to target and eradicate cancer.
She thought this was her last chance.
A week after receiving treatment, her lymph nodes shrunk. Within three months, there was no evidence of the disease.
Today, she’s cancer free — and sharing her experiences to help us better meet the needs of patients like her in our cell and gene therapy trials.
Lives can change when you design cell and gene therapy trials with speed and precision.
- Utilize the right experts, focused on the right indications
- Access even hard-to-find patient populations
- Pinpoint the perfect sites for your trial
- Satisfy global regulations, to get your treatment to market safely and quickly
What we do, we do

Our Experts
Our cell and gene therapy specialists collaborate to help get your innovative treatments to patients like Robyn faster.

Our Experts
Chris Learn, Ph.D., PMP
Vice President, Cell and Gene Therapy, Center of Excellence
With 20+ years of trial execution and team management experience, Chris leads development for our cell and gene therapeutic area. He reinforces our patient-first focus to help ensure your trials are designed to meet patient needs.
"Being able to capture and understand the patient’s perspective — not just as a patient, but as a person — and use their insight to guide my decisions is what it means to work With Heart™."
Our diverse experiences ensure you get the expertise you need, no matter the indication.
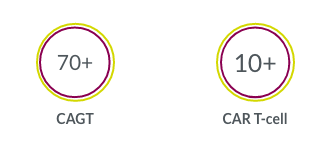
Our experience with CAGT clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Europe
- APAC
- Middle East & Africa
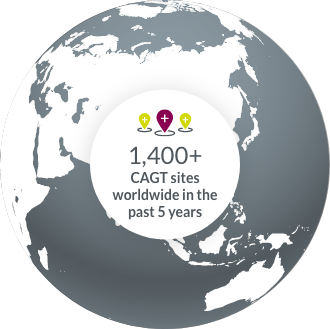
Access to global EMR data enables us to pinpoint the perfect sites for your trial.

Our cross-functional team overcomes any technical, logistical, and strategic challenges.
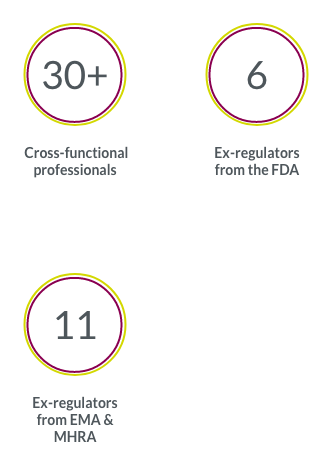
Our highly tailored recruitment and retention strategies drive access to even hard-to-find patient populations.

What can we do to help you change patient lives?
See CAGT capabilities Visit all therapeutic areas Explore CAGT careers
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
CloseWe focus on patients, because they inspire us to deliver better trials, faster than ever. So we can make a difference for more patients like Robyn.
Who we are,
Parexel is proudly among the world’s largest
clinical research organizations
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 24,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind, to make clinical research a care option for anyone, anywhere.



