The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
ClosePatient Story
Sara's only symptom was fatigue. But after some routine tests, she was diagnosed with breast cancer.
Pause Background MotionNow she was the one in need of care — joining the 3.8 million women in the US and many others around the world impacted by breast cancer.
For years she'd worked at a hospital, onboarding new doctors and making care possible for others.
She started radiation. A new, promising chemotherapy treatment was also available, so she joined the clinical trial.
That decision changed her life.
Today, she’s cancer free — and works at Parexel to help ensure our trial sites are prepared to meet the needs of patients like her.
Lives can change when you design oncology trials with speed and precision.
- Utilize the right experts, with the right specialization
- Find the patients you need and earn their trust
- Satisfy global regulations to get your treatment to patients safely and quickly
- Design studies and endpoints with market access in mind
What we do, we do

Our Experts
Our oncology specialists collaborate to help get your treatment to patients like Sara faster.

Our Experts
Gwyn Bebb, M.D., BM, BCh, Ph.D.
Senior Vice President, Global Therapeutic Area Head – Oncology
Gwyn is a licensed medical oncologist with more than 20 years’ experience in academia and the clinical research and biotechnology industries. At Parexel, he leads the research and development of new cancer treatments for biopharmaceutical customers worldwide. His depth of expertise in oncology drug development and proven experience in leading cross-functional teams positions him as an invaluable resource for customers and a natural leader of our global oncology franchise.
Our oncology team is 1,000+ strong, with experience across the oncology continuum, to match the needs of your trial.
Advanced modeling and simulation allow us to predict drug effects in advance , saving time, money, and resources.
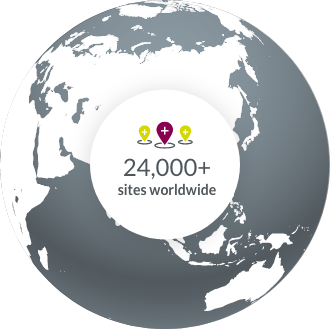
Our experience with oncology clinical trial sites around the world allows us to accelerate study start-up.
- North America
- South America
- Europe
- APAC
- Middle East & Africa
Our application of real-world data allows us to find the right study participants fast.

Our global regulatory team keeps everything running smoothly.
And our patient-first approach results in deeper, more relevant insights for trial design and execution.
TACTICS
BENEFITS MAY INCLUDE
What can we do to help you change patient lives?
See oncology capabilities Explore oncology careers Contact us
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
CloseWe focus on patients, because they inspire us to deliver better trials, faster than ever. So we can make a difference for more patients like Sara.
Who we are,
Parexel is proudly among the world’s largest
clinical research organizations
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 24,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind, to make clinical research a care option for anyone, anywhere.






