Medical Communications
Rely on our expert guidance to effectively communicate complex scientific and medical information to diverse audiences.
Rely on our expert guidance to effectively communicate complex scientific and medical information to diverse audiences.
Communicating scientific and related data to your various stakeholders requires a thoughtful, integrated, and engaging approach. We combine our comprehensive portfolio of medical communications services with the power of Parexel’s broader therapeutic, clinical development, patient engagement, real-world evidence, health economics, market access, and regulatory expertise to provide you with meaningful insights into the needs of all your key stakeholders. This enables us to create a robust strategic communication framework and integrated communication plan to ensure meaningful engagement with thought leaders, healthcare providers, patients, and payers on their journey of information discovery.
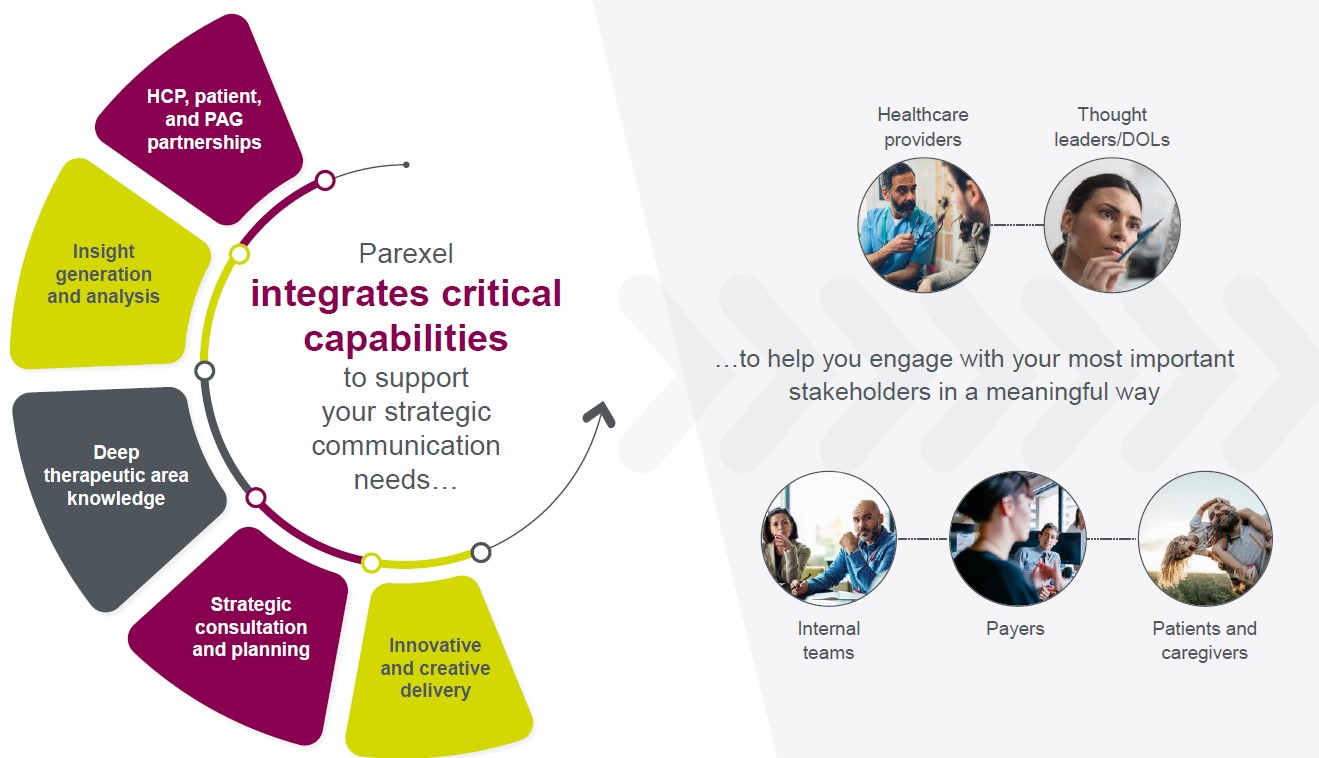
To do this, we employ world-class Medical Affairs strategists supported by highly skilled PhD, PharmD, and MD writers and content developers who are passionate about their craft; seasoned medical editors; meeting logistics experts; and creative, digital and design specialists who enhance the educational experience and bring the science to life.
Parexel Medical Communications is a full-service agency, providing Medical Affairs solutions across the entire product development lifecycle, with experience in every major therapeutic category, many rare diseases, and novel cell and gene therapies.
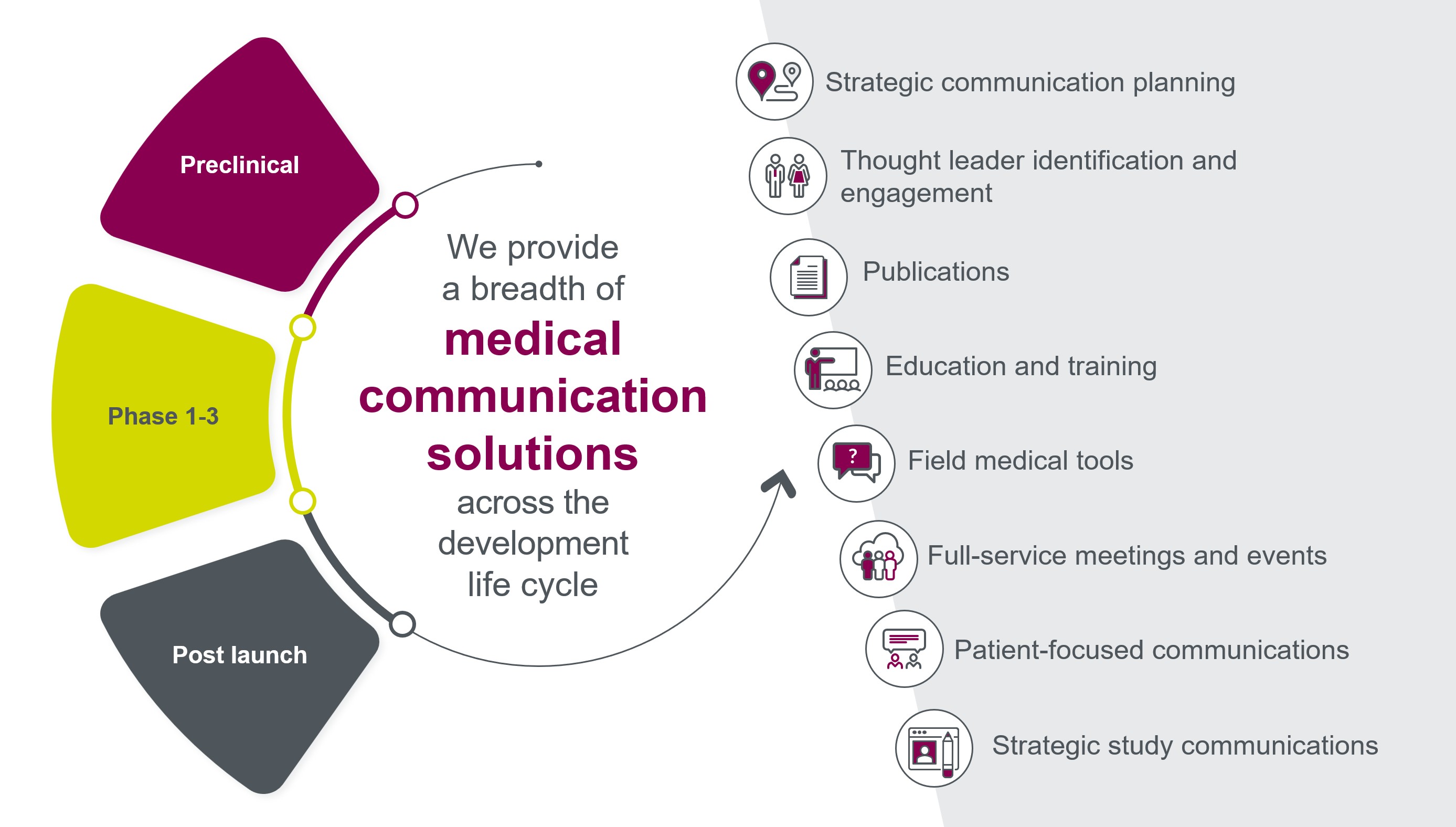
As part of a larger CRO, we take an integrated evidence generation planning approach that gives us a unique perspective, and allows us to deliver bigger and bolder resources, tools, training, and events, rich with creativity and innovation while ensuring scientific rigor.
Our experts also provide comprehensive strategic communications support for site, investigator, and patient engagement for clinical and RWE studies.
Contact us at +MedCom-Customer-Strategy@parexel.com
Our experts
Related Insights