With the introduction of new health technology assessment (HTA) regulation in the European Union (EU), the joint clinical assessment (JCA) aims to remove multiple country-specific assessments and to improve the functioning and transparency of a single market for health technologies.
The evidence required by the new appraisal process is submitted only once, in a JCA dossier at EU-level. In this context, the relative clinical effectiveness of drugs compared to the standard of care, with particular attention to patient relevance, is gaining importance.
Outlining the PICO(s) timing and requirements
JCA will evaluate the clinical effectiveness of all new treatments under the PICO framework, which is: Population, Intervention, Comparator, Outcomes.
Each indication is likely to require multiple PICO(s), which can be 27 or higher depending on the patient population/sub-populations of interest and the comparators considered as “standard of care” across the member states.
There is a limited timeframe for companies to develop and submit the dossier with the evidence on multiple PICOs covering the requirements of the 27 EU member states, after the final JCA scope:
- ~90 days for submission
- Note that this is an average, so there may be scenarios where companies would need to submit their dossier significantly earlier for fast-track applications, resulting in less preparation time
- 7 – 30 days to respond to further requests on data submitted, analyses or additional evidence
- Consider too that revision may be needed if the indicated patient population is restricted (as submission to JCA must be done before the Committee for Medicinal Products in Human Use (CHMP) decision, so there is a risk of repeat submission to JCA)
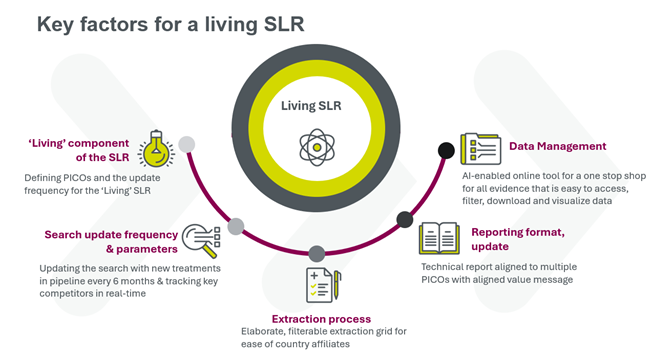
Proactively manage evidence and timeline requirements with a living SLR
To meet JCA evidence requirements, within the required timeframes (including accelerated scenarios for fast-track applications) companies need to be prepared. A living systematic literature review (SLR) can support the evidence needs of all potential PICOs, by maintaining a literature review that is updated in real time and identifies new evidence when it’s published.
At Parexel, our living SLRs are built on an AI-enabled platform, helping to deliver:
- Up-to-date evidence
- One consolidated database to manage the body of evidence for multiple PICOs
- Ability to segment evidence by patient sub-groups and comparators to meet the member state requirements
- Ease of conducting meta-analyses and network meta-analyses
- Optimized development of country-specific value messages and dossiers
Our Access Consulting team is always available for a conversation on living SLR development to support your value narrative for JCA submissions.



