Optimizing engagement with the Institute for Clinical and Economic Review (ICER) through early economic model development
In 2023, ICER updated its value framework to include a more holistic perspective of value demonstration. Key updates include:
Clinical trial diversity
- Pivotal trials based on participant diversity in race/ethnicity, sex, and age.
Patient perspective in clinical trial design
- Manufacturers should detail their patient experience data collection techniques and explain how they determined the most impactful outcomes for patients.
- Assessing patient utility scores and time-use data to evaluate productivity outcomes (return to full work and family activities).
- Patients will be surveyed to capture other value considerations.
IRA and CEA model
- ICER will engage with stakeholders to include generic competition and Medicare drug price negotiation, influenced by the Inflation Reduction Act (IRA).
Generalized risk-adjusted cost-effectiveness (GRACE) framework
- In a GRACE model, severe conditions are valued more than in standard cost-effectiveness methods.
- This may lead to increased emphasis on patient-reported outcomes that address concerns over the discriminatory potential of the QALY metric.
For drugs selected by ICER, recommendations from its cost-effectiveness models continue to influence payer coverage decisions.
However, preparation is key. If selected for ICER review, manufacturers only have five months to respond. Parexel analysis indicates that not being prepared for ICER reviews has resulted in an unfavorable outcome in 78% of the cases.
Early economic model development for ICER evaluation readiness
Early economic models that can be adapted to ICER-like models can be invaluable for evaluation readiness. These models provide decision-makers with early insights into key drivers, evidence gaps, and the potential economically justifiable price.
Parexel Access Consulting supports manufacturers in developing early economic models to prepare and plan for ICER review or, if selected, respond to ICER’s evaluation. With expertise in HEOR modeling and payer/HTA interactions, backed by a panel of technical experts, Parexel develops a systematic, evidence-driven approach to increase the likelihood of a favorable outcome – as well as bolster value narrative for payer engagement.
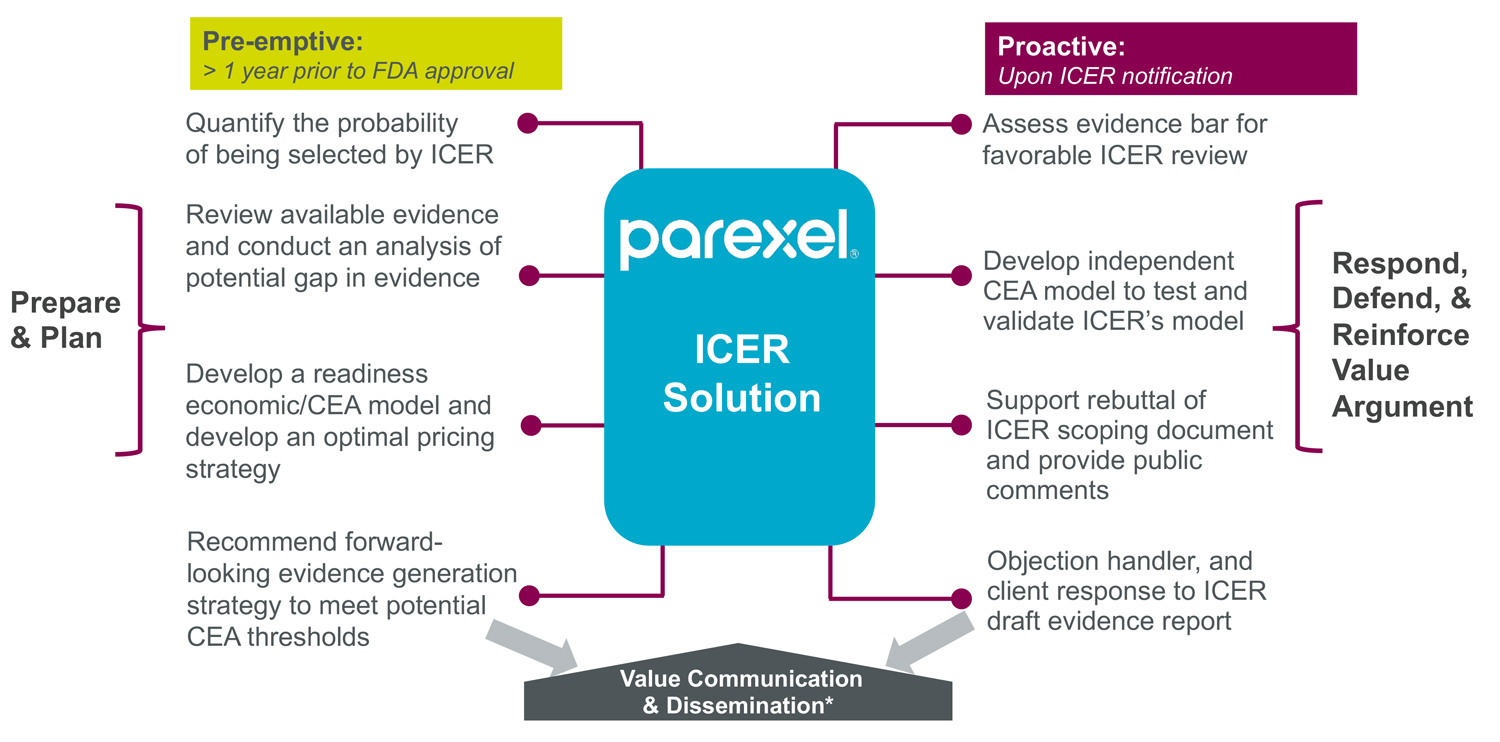
Parexel’s approach for ICER review and evaluation readiness
Serving as guideposts for shaping the drug pricing landscape, we anticipate growing acceptance of such value assessments among commercial payers in making coverage and formulary decisions. As such, Parexel strongly advises manufacturers to embrace ICER-like early economic modeling to present value outputs for their drugs.
Our Access Consulting team is always available for a conversation to develop early economic models and support your engagement with ICER.
Our experts in access consulting



