Preparing for joint clinical assessment (JCA) scenarios in the European Union (EU)
JCA (joint clinical assessment) was established in the EU as a single, transparent evaluation to bring innovative medicines to patients, faster. JCA will evaluate the clinical effectiveness of all new treatments under the PICOs framework, which is:
- Population
- Intervention
- Comparator
- Outcomes
However, different activities are required depending on the country-level response to JCA evaluation, and how each country will implement the EU health technology assessment (HTA) into their legislation and processes.
After JCA evaluation, the country-level response includes three potential outcomes:
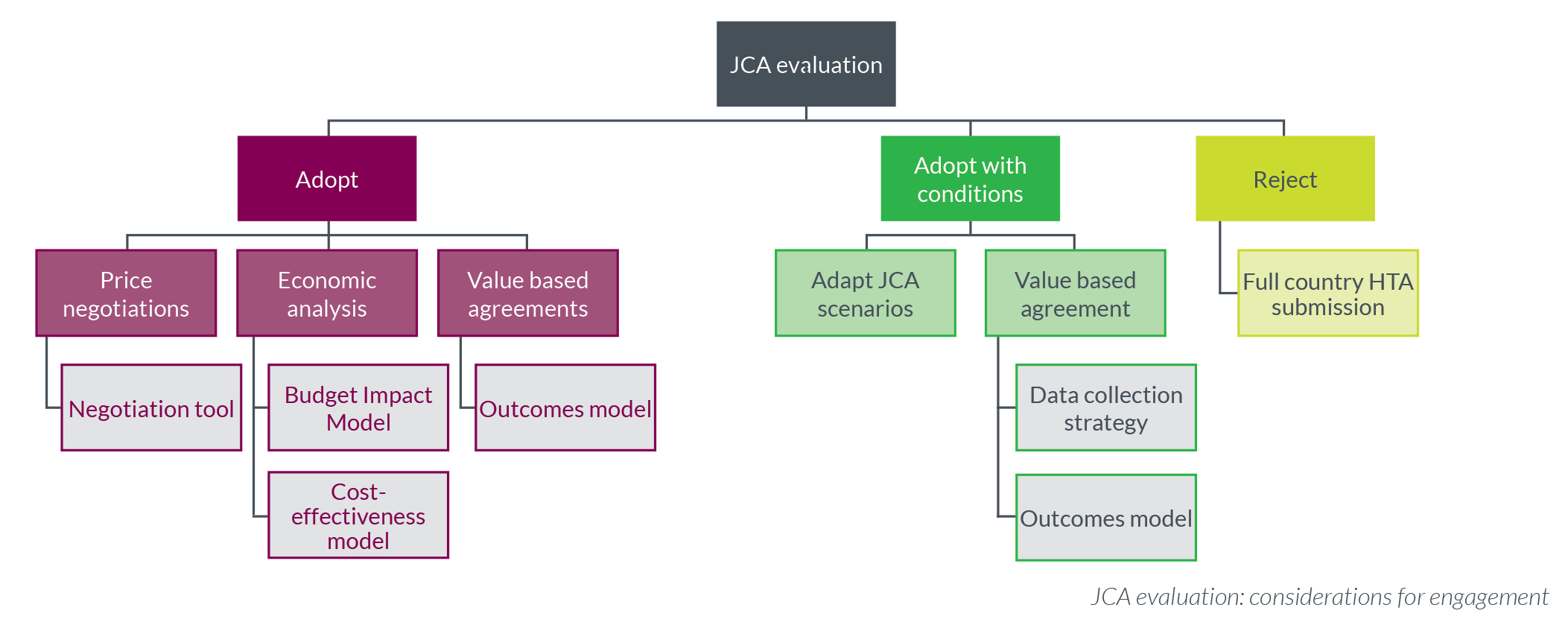
Different activities are required for each outcome. These are the questions, considerations, and processes to consider for each:
To adopt JCA evaluations, countries will need to consider economic frameworks to ensure value for money:
- What economic considerations need to be incorporated into the country's HTA process to ensure that the appropriate pharmacoeconomic evaluation is considered?
- What process needs to be implemented to ensure that the appropriate pharmacoeconomic evaluation is being used? For example: cost-utility or cost-minimization
- Who are the stakeholders that need to be engaged to ensure that the appropriate methodology and technical skills are being utilized?
What if the JCA evaluation needs to be adopted with conditions to ensure that it represents the country’s healthcare system?
- What data or evidence is required for adaptation of the JCA to be adopted by the country?
- What real-world evidence or real-world data is required to ensure that a value-based agreement is appropriate to measure the clinical and economic value of the new treatment in the country’s healthcare system?
- What process needs to be implemented to ensure that the appropriate data collection methodology is used to synthesize evidence to reduce the uncertainty?
- Who are the stakeholders that need to be engaged to ensure that the correct methodology and technical skills are being utilized?
If the JCA evaluation does not represent the country’s healthcare system and cannot be adopted, what is the next step?
- An assessment of the existing in-country HTA system to continue to evaluate new medicines
Considering the requirements to navigate the JCA, do you have the internal governance and skill sets to respond to these scenarios effectively? Delays through the JCA processes, assessment and local adoption will have implications for patients and commercial teams.
Our Access Consulting team is always available for a conversation to help you navigate your preparation for JCA.
our experts in access consulting



