The Inflation Reduction Act of 2022 (IRA) empowers Medicare to negotiate prices of some products, impacting drug manufacturers’ pre-launch and post-launch commercial strategies.
IRA impacts:
- Clinical development and business development strategy/asset evaluation
- Evidence development
- Portfolio optimization
- Pricing strategy
As part of pricing negotiations, Centers for Medicare and Medicaid Services (CMS) will offer a maximum fair price (MFP) to the drug manufacturer. The MFP is based on nine parameters:
| Manufacturer-specific data: | Evidence about alternative treatments: |
|---|---|
|
|
MFP offers could be 40 – 75% lower than list price, based on the parameters above.
Prepare for IRA negotiations with a robust evidence package submission, including independent determination of MFP
To bring transparency and a focus on value to both the launch price determination process and a basis for post-launch MFP negotiations, Parexel can provide an independent determination of MFP to serve as a foundational reference.
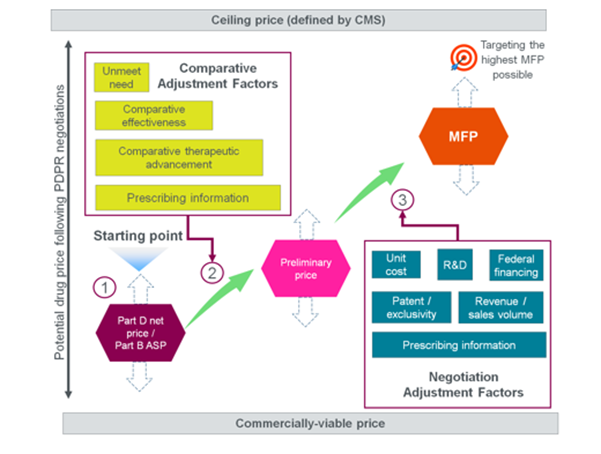
Parexel’s framework for MFP determination sequence
Our bespoke solution is built on expertise in HEOR modeling and payer/HTA interactions and supported by technical experts. Our Access Consulting team is always available for a conversation to support your preparation for IRA negotiations, including independent determination of MFP.



