Enabling GxP compliance for drug development and manufacturing
As regulatory environments rebound from the impact of COVID-19, on-site agency inspections are regaining momentum, increasing scrutiny on global supply chains.
To address these challenges, we partner with organizations of all sizes to build cultures of compliance, mitigate risk, and restore brand and regulatory confidence. Through onsite and remote audits (pre-approval inspections and for-cause), training and remediation management, we deliver a range of projects each year, including:
- 100+ GxP audits
- 20+ Form 483/Warning Letter remediation projects
- 35+ Risk mitigation advisory projects
GxP compliance consulting
Compliance breaches have consequences for patients awaiting treatment, as well as on manufacturing operations and throughout the supply chain. Our team of former regulatory inspectors and industry experts work closely with companies to ensure uninterrupted supply, from development phases to manufacturing and market availability of vital products.
Enhance your operations, maximize efficiency and ensure regulatory adherence with our compliance and risk management services, including:
Strategic advisory
Whether it’s nimbly responding to an issue or building proactive compliance strategies, our goal is to help you confidently navigate every aspect of risk mitigation, and crisis and compliance management. We do this by tailoring solutions that make sense for your situation, your supply chain, and ultimately, the patients you serve.
Auditing
Identify and resolve compliance challenges with our detail-oriented auditing services. Our auditors conduct thorough GxP audits, including mock pre-approval inspections (PAIs), verification audits, and effectiveness checks. By proactively identifying areas of improvement, we help you mitigate risks and maintain regulatory compliance.
Our team of experts offer guidance on Good Distribution Practice (GDP), Good Manufacturing Process (GMP), Good Clinical Practice (GCP) Quality Management Systems (QMS), and effective management control.
Data integrity
Ensure data integrity and build confidence in the quality of your data. Achieve data integrity through comprehensive audits, expert analysis, targeted training and streamlined procedure implementation – as well as staff interviews, internal investigations, and fraud evaluation.
Inspection support
Minimize the risk of compliance-related rejections and ensure a smooth regulatory inspection process. Our comprehensive range of services to prepare you for your PAI include mock interviews, readiness assessments and application conformance reviews. With front/backroom support and follow up assistance, our focus is supporting you to achieve successful outcomes.
Technical writing
Accurate and comprehensive SOPs, regulatory agency correspondence, audit responses, strategic technical reports, and annual product reviews can save time and minimize the risk of compliance issues. Meet all the related regulatory requirements with documentation that precise, thorough, and aligned with industry standards.
Consent decrees and warning letters
Navigate consent decrees and warning letters with our strategic and operational compliance support. When faced with unexpected regulatory crises, we provide swift, well-balanced, and comprehensive responses to address the situation effectively. Starting with root cause identification, and delivering thorough remediation planning, implementation, and execution, we help you overcome regulatory challenges and restore compliance.
Risk-based quality management with advanced analytics
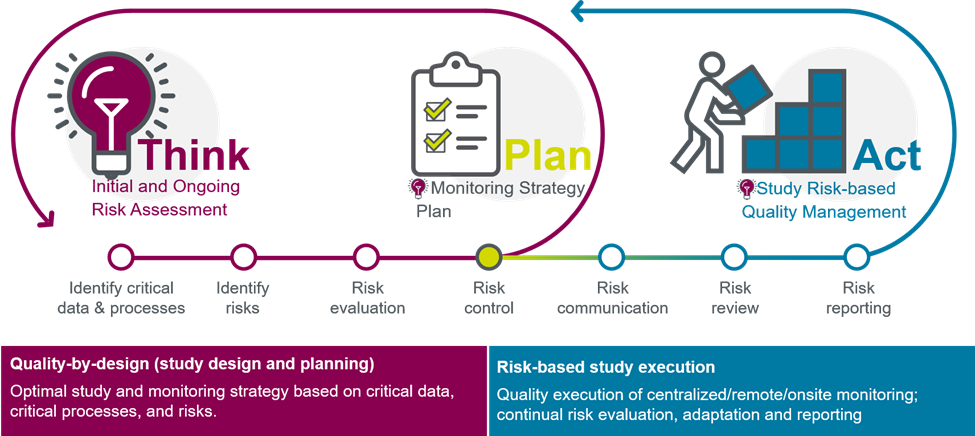
Ever-increasing trial complexity, expanded data sources, surging data volumes, and a host of new delivery models require fundamentally different approaches to how we collect and manage clinical trial data.
Parexel offers risk-based quality management services (RBQM), keeping you ahead of clinical development data challenges with essential monitoring that has become a requirement in today’s high functioning clinical trial ecosystem.
We help you identify, capture, and manage such data quality issues at the earliest stages of emergence — before potential risks escalate to liabilities. This approach forms the basis of a fit-for-purpose monitoring strategy, using study risk assessments, complexity, country requirements, site monitoring, and capabilities as key considerations. A continuum of models includes:
- Central monitoring
- Remote monitoring variations
- Traditional onsite monitoring
Our advanced analytic capabilities leverage historical data across hundreds of trials to model predictive scenarios and categorize risk by severity of impact. AI techniques complement clinical development and therapeutic knowledge to offer an unprecedented level of sensitivity when characterizing trends of importance or potential compliance issues.
- Preventable quality issues can be avoided with agile decision-making
- Known and unknown risks can be rapidly detected on both critical and non-critical data
- Monitoring resources can be prioritized based on data insights rather than the traditional 100% source data verification (SDV) approach
We look at the world a bit differently post-pandemic, with compliance and risk viewed in a new reality. Our services are designed to help you strike the right balance, providing a cost-effective regulatory solution to decisively manage risk and respond to crises.
Our experts
Browse our latest insights
Want to hear from our experts on the latest industry topics? Visit our Insights Center to read, watch, and listen.



