The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
ClosePatient Story
- Rare Diseases
- Oncology
- Inflammation & Immunology
- Cell & Gene Therapy
- Neuroscience
When Austin was 3 years old, his parents realized something was wrong.
DMD weakens the muscles until the patient can't walk and greatly shortens their lifespan. There's no cure.
After multiple falls, concussions, and a broken arm, they discovered he had Duchenne muscular dystrophy, a condition diagnosed in 20,000 children globally each year.
Through clinical trials, however, Austin found treatment to slow the disease and live a better life.
Today, he's a college student, co-founder of the nonprofit One Rare, and a member of our Patient Advisory Council — helping us better meet the needs of rare disease patients.
Lives can change when you design rare disease trials with care and precision.
- Become a study of choice in patient communities
- Expedite startup and accelerate timelines
- Satisfy global regulations to get your treatment to market safely and quickly
- Find the right experts, focused on the right indications
What we do, we do

Our Experts
Our rare disease specialists collaborate to help get your treatment to patients like Austin faster.
MORE EXPERTS
Stacy Hurt, M.H.A., M.B.A.
Chief Patient Officer
Chris Learn, Ph.D., P.M.P.
Senior Vice Presiden...
Angela Qu, M.D., Ph.D.
Senior Vice Presiden...
Wyatt Gotbetter
Senior Vice Presiden...
Martin Roessner
Corporate Vice Presi...
Peter Kiely, M.D.
Vice President, Tech...
Steve Winitsky
Vice President, Tech...
Rachel Smith
Executive Director, Rare Disease Center of Excellence
With 10+ years of experience in rare diseases and cell and gene therapy trials, Rachel leads our global, cross-functional Center of Excellence for Rare Diseases. She supports strategies to deliver your projects effectively, ensuring the patient perspective is included every step of the way.
Close
Our Experts
Rachel Smith
Executive Director, Rare Disease Center of Excellence
With 10+ years of experience in rare diseases and cell and gene therapy trials, Rachel leads our global, cross-functional Center of Excellence for Rare Diseases. She supports strategies to deliver your projects effectively, ensuring the patient perspective is included every step of the way.
"We need to develop products that meet the needs of the patient. That’s why we work so closely with them, focusing on improving their quality of life."
Our diverse rare disease experiences ensure you get the expertise you need, no matter the indication.
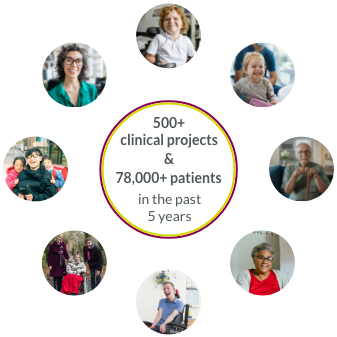
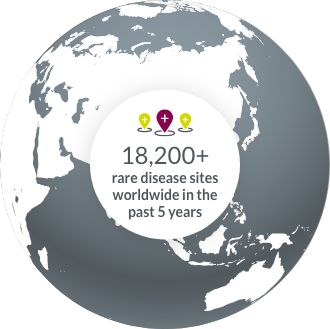
Our collaboration with rare disease clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Europe
- APAC
- Middle East & Africa
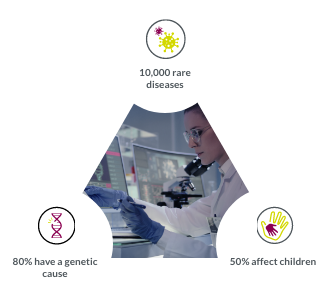
Our field-leading genetic specialists customize your endpoints by age, genotype, and phenotype for better data.
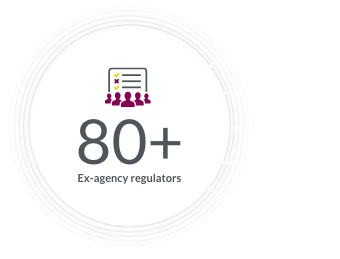
Our global regulatory team helps keep everything running smoothly, with expertise in breakthrough therapy designations and more.
And our patient-first approach results in deeper, more relevant insights for trial design and execution.
TACTICS
BENEFITS MAY INCLUDE
What can we do to help you change patient lives?
See rare diseases capabilities Visit all therapeutic areas See rare diseases career openings
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
CloseWe focus on patients, because they inspire us to deliver better trials, faster than ever. So we can make a difference for more patients like Austin.
Who we are,
Parexel is proudly among the world’s largest
clinical research organizations
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind, to make clinical research a care option for anyone, anywhere.







