The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
ClosePatient Story
- Immunology
- Cell & Gene Therapy
- Neuroscience
- Endocrine & Metabolic
Tina had lost her father to Crohn's disease. Now she faced her own diagnosis.
Then came the diagnosis. Finding out she had Crohn’s disease, one of two IBD syndromes that affect 10 million people worldwide, wasn’t a surprise considering her family’s history with the disease. But it derailed her life.
Tina was in her early 20s. She lived in New York City and was building a promising future for herself in finance.
She spent the next 10 years trying countless medications and underwent 20 surgeries. Nothing worked.
Then, finally, she enrolled in a clinical trial.
Within months, she was in remission—and has been ever since.
Today, she’s a patient advocate, sharing her experiences to help keep our clinical trials focused on the needs of patients like her.
Lives can change when you design inflammation and immunology trials with agility, precision, and care.
- Keep up with urgent clinical demands
- Utilize the right experts, with deep I&I experience
- Help participants with chronic conditions feel safe and cared for
- Get seamless, end-to-end support across indications.
What we do, we do

Our Experts
Our inflammation and immunology specialists collaborate to get your treatment to patients faster.

Our Experts
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Mwango brings over 20 years of clinical and regulatory drug development experience to her role at Parexel, including more than 16 years at the U.S. Food and Drug Administration (FDA). She provides strategic and technical guidance on various regulatory and clinical aspects of drug development, leveraging her expertise in clinical review, pharmacovigilance and post-approval safety requirements across multiple therapeutic areas. Mwango is a critical asset for our customers, helping them navigate the changing regulatory environment on their development journeys to deliver important new treatments to patients in need across the globe.
Our I&I team is 13,100+ strong, with experience across indications, to match the needs of your trial.

Our diverse project experiences and expertise in patient recruitment and engagement result in fast, efficient trials.

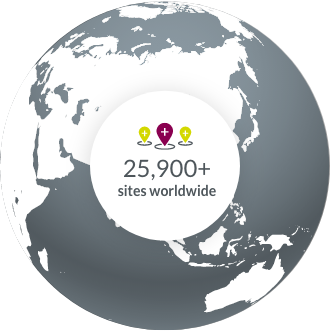
Clinical trial sites around the world allow us to accelerate study startups.
- North America
- South America
- Europe
- APAC
- Middle East & Africa
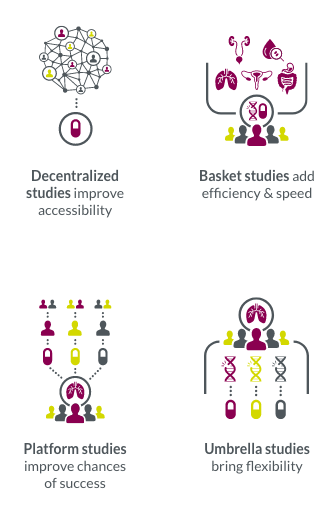
Innovative trial designs allow us to optimize your trials for maximum impact.
And our patient-first approach results in deeper, more relevant insights for trial design and execution.
TACTICS
BENEFITS MAY INCLUDE
What can we do to help you change patient lives?
See I&I capabilities Visit all therapeutic areas Explore I&I careers
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
CloseWe focus on patients, because they inspire us to deliver better trials, faster than ever. So we can make a difference for more patients like Tina.
Who we are,
Parexel is proudly among the world’s largest
clinical research organizations
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 24,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind, to make clinical research a care option for anyone, anywhere.



