The Inflation Reduction Act (IRA), enacted in August 2022, aims to enhance the affordability and accessibility of innovative drugs for Medicare beneficiaries. It allows Centers for Medicare and Medicaid Services (CMS) to negotiate a maximum fair price for therapies covered under Medicare Part B and D.
To be considered for price negotiation under the IRA, a drug must meet several criteria:
- It must be a single source product
- It should not have generic or biosimilar competitors
- It must be covered under Medicare Part D or Part B
- It should have a high annual expenditure in the US
Under the Act, small molecule drugs are eligible for negotiations seven years from FDA approval, while biologics are eligible after 11 years.
Preparing for IRA negotiation
Pharmaceutical companies should anticipate potential IRA negotiations and prepare accordingly if their product is selected. This includes developing a value-focused dossier to support the drug pricing, highlighting:
- Product value including FDA-approved indications, dosing flexibility and guideline recommendations
- Clinical value including clinical trial data, comparative effectiveness, and evidence from real world scenarios
- Economic value including budget impact and reductions in healthcare-related resource use, cost-effectiveness and societal value
- Unmet need in terms of epidemiology, humanistic burden, societal burden, economic burden and treatment patterns
AI-powered approaches streamline the creation of value-focused dossiers to meet CMS requirements
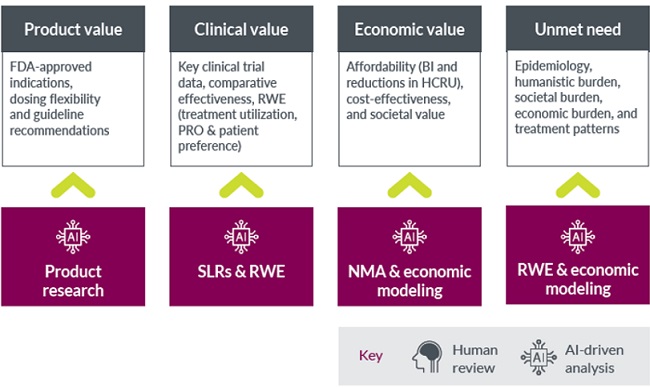
At Parexel, we utilize AI-powered approaches to support pharmaceutical companies’ readiness for CMS negotiations by streamlining evidence generation and analysis. AI provides valuable support, including:
- Product research to identify treatment guidelines recommendation to support product value
- Systematic literature reviews to identify evidence on various aspects of the product such as: efficacy and safety from clinical trials and real-world sources, treatment utilization and pattern, patient reported outcomes, patient preferences, and healthcare related resource utilization
- Feasibility assessment and network meta-analysis to establish comparative effectiveness of the product
- Real world data analytics to bring in insights into clinical effectiveness, safety, and healthcare resource utilization patterns from the use of the product in real world setting
- Economic modelling to establish cost-effectiveness of the product, impact on budget for the payers and the economic value to both payers and society
Ethical AI implementation with human oversight and control
Aligned with Parexel’s six principles of an ethical approach to AI solution development, we understand the importance of skilled human oversight when using AI. Our “human-in-the-loop” approach combines AI-driven evidence gathering and analysis with human expert oversight. Humans review, interpret, and contextualize AI outputs, ensuring critical supervision. This collaboration mitigates potential biases and misinterpretations, resulting in comprehensive, efficient, and nuanced analyses that align with human expertise and judgment.
By leveraging AI in research and analysis activities to prepare for IRA-related requirements, pharmaceutical companies can gather more comprehensive, accurate, and timely information to support their value proposition during CMS negotiations. This AI-enhanced approach can lead to more robust evidence packages and potentially stronger negotiating positions.
Our Access Consulting team is always available for a conversation on AI-enabled solutions to support your evidence generation and analysis. Please get in touch.
Our experts
Sugandh Sharma, MSc
Executive Director, Health Economics and Outcomes Research, Access Consulting
Jackie Vanderpuye-Orgle, Ph.D.
Senior Vice President, and Global Head of Advanced Analytics, RWE & HEOR
Sangeeta Budhia
Senior Vice President, Pricing & Market Access
Brian Duda
Principal Consultant, Pricing & Market Access



