Harness the power of whole-study solutions
We design and deliver studies that ensure patient safety, data integrity, regulatory compliance, and scientific rigor. Our integrated strategies address these priorities and more, allowing us to reduce burdens, mitigate risk, and operate efficiently within timelines and budgets.
Delivering clean data faster so you can reach your milestones sooner
Integrated Data Delivery is a clinical study delivery ecosystem that drives a holistic “Touch Data Once” strategy of risk-based data monitoring & cleaning. This ensures that the appropriate experts are engaged with the most relevant data exactly when it matters.
By redesigning the data architecture and sequence we’ve streamlined cleaning and monitoring, reduced redundancy, and ensured the right people touch the right data at the right time, resulting in:
- Improved site experience; less interruption to their practice
- Shortened time to major deliverables
- Earlier data-driven decisions and actions
- Better insights for quality, compliance and reduced risk
- Better sponsor oversight
Our solution experts
Deb Tatton
President, Global Clinical & Data Operations
Mike D’Ambrosio
Senior Vice President and Global Head, Real World Research
Paul Bridges, Ph.D.
President, Consulting
Andreas Lysandropoulos, M.D., Ph.D.
Franchise Head, Neurosciences, Ophthalmology, and Rare Diseases
Sanjay Vyas
President, Safety & Logistics and Country Head of India
Katie Connelly
Senior Vice President, Global Head of Regulatory Affairs
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Our solution experts
Deb Tatton
President, Global Clinical & Data Operations
Mike D’Ambrosio
Senior Vice President and Global Head, Real World Research
Paul Bridges, Ph.D.
President, Consulting
Andreas Lysandropoulos, M.D., Ph.D.
Franchise Head, Neurosciences, Ophthalmology, and Rare Diseases
Sanjay Vyas
President, Safety & Logistics and Country Head of India
Services
- Phase IIb-IV clinical trials
- Real-world evidence
- Model-informed drug development
- Protocol-driven, customized site solution strategy
- Regulatory strategy, submissions, compliance, and outsourcing
- Market access strategy and delivery
- Clinical development technology optimization
- Clinical trial supply and logistics
- Patient inclusion
- Medical communications
- Drug safety & pharmacovigilance
- Integrated evidence generation planning
Phase IIb-IV clinical trials
Today, as the design and conduct of clinical trials continue to evolve, decision-making needs to be informed by strong expertise in biostatistics, data analysis, and data management. This knowledge is essential to ensure patient safety, data integrity, and operational feasibility as flexible protocols become the norm.
At Parexel, we offer this depth of expertise in supporting protocol design, trial execution, site management, and all the way through to market strategy and commercialization. We take a risk-focused approach, continually assessing the tangible risk of the trial so we can control it, adapt to it, and keep everything running smoothly.
Real-world evidence
Explore the impact of a therapy or disease in a real-world setting, generating data that can support your value proposition without additional burden to the patient. Our real-world evidence (RWE) strategies help reassure payers, regulators, providers, and patients that your clinical results are reflective of what will be evidenced in real-world practice, helping demonstrate value before, during, and after launch.

To access the patients your study needs, we incorporate decentralized trial (DCT) elements into our proposals.
Model-informed drug development
Quantitative clinical pharmacology, pharmacometrics, and programming combine to create a strategic approach in clinical development, optimizing the match between patients, treatments, and dosages.
This integrated approach leverages pharmacokinetic and pharmacodynamic data to guide trial design and drug selection, ultimately reducing development time and costs while maximizing patient benefits and minimizing side effects.
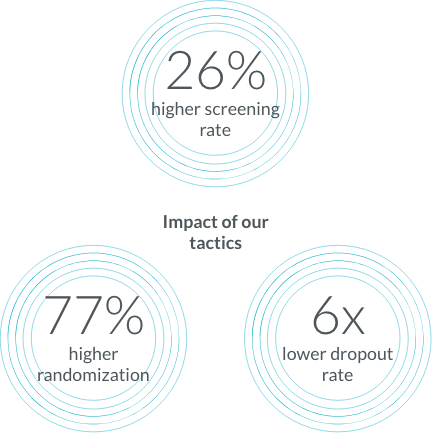
Over the course of 90+ dermatology studies that enrolled 30,000+ patients our patient education and engagement tactics led to better screening rates, optimization, and a lower dropout rate.
Protocol-driven, customized site solution strategy
In response to the increasing complexity of trial protocols and the pressures faced by sites, we accelerate study start-up by helping you find the right sites, patients, and partnerships. Our multidisciplinary Launch Excellence Office aims to meet the specific needs of your study, offering study planning, critical-path oversight, and delivery execution as key elements. We also engage in partnerships with groups offering a broad range of perspectives, so we can design protocol and site-selection strategies with the biggest possible impact in mind.

Our Site Alliance Network gives you access to more sites, diverse populations, and investigators so you can be confident in your site selection strategy.
Regulatory strategy, submissions, compliance, and outsourcing
At this pivotal point in the development process, take control of your regulatory pathway and reduce compliance risks to simplify and accelerate your treatment’s journey to patients.
Our global team of former regulators helped set the standards you’re striving to meet. We’ll help you optimize your regulatory pathway to maximize the value of your product.
Our former FDA, EMA and NMPA regulators, supported by 1,300+ colleagues in local regulatory teams help you manage global complexity in changing regulatory environments.
Market access strategy and delivery
Even with a flawlessly executed clinical trial and a strong regulatory strategy, there’s no guarantee of a successful product launch. Without robust commercialization planning, patient access and return on investment (ROI) may be limited.
We’ll help with that, by delivering a product market access strategy that merges clinical and development plans with regulatory and health economic strategies.

Our market access team has the experience and expertise to help you optimize ROI and patient access, having completed 150+ market access strategy projects in the past 10 years.
Clinical development technology optimization
To make the most of your valuable data, we design fit-for-purpose technology solutions — suites that capitalize on our own mature systems along with best-in-class technology from industry-leading vendors. Each solution is engineered and implemented by experts to address the specific needs of your trial and team.
Our solutions go beyond data collection. We learn about the decisions your team will face, then create ways to curate, visualize, and monitor your data so that you can make those decisions quickly and with confidence.

We won the 2022 Frost & Sullivan Global Customer Value Leadership Award for excellence in planning, operationalizing, and delivering decentralized trials (DCT) worldwide via leading-edge technology.
Clinical trial supply and logistics
Whether conducting a small regional or a complex global trial, you need a robust supply chain network that includes country-specific knowledge and a close connection to local authorities. We provide the knowledge, systems, and connections to achieve end-to-end clinical trial supply chain management. We’ll help you build a supply chain that sails through international pressure points and delivers drugs to patients and trial sites right on schedule.
To ensure that our clients are free to conduct trials around the world, we operate a hub-and-spoke network of depots that seamlessly move drugs and ancillary supplies to investigator sites. Our skilled team of trade compliance professionals will handle customs declarations, apply for permits, and manage interactions with local governments so you can focus on running the best trial for your patients.

We’ve won multiple Asia-Pacific Bioprocessing Excellence Awards, including 2021 Best Clinical Supply Chain, 2020 Best Supply Chain Digitization, and 2019 Most Robust CRO Supply Chain.
Patient inclusion
Clinical trials have often been an exclusive space, rather than an inclusive one, with serious consequences for patients in underrepresented groups. At Parexel, it’s our mission to change that. We’ve gone straight to patients, caregivers, physicians, and community leaders to identify factors that prevent patients from enrolling in clinical trials.
No matter what steps you’ve taken to address equity and inclusion in your trial design process, we’re ready to work with you. We’ll help you design trials that include patients who otherwise might not have been able to participate due to issues such as time, finances, and transportation. By focusing on inclusion, you’ll help more patients while developing drugs and therapies that can work for all.
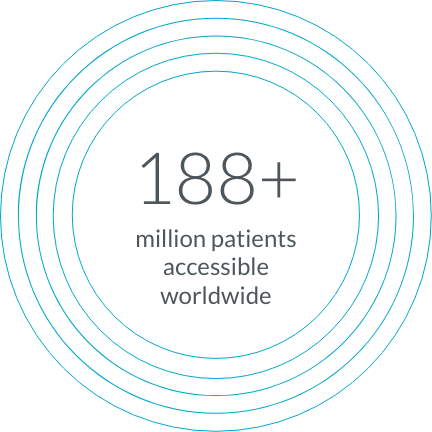
Through TriNetX Live, we’re able to access a network of millions of patients in more than 30 countries, so you can access the diverse populations you need.
Medical communications
Engage the people who matter most. At Parexel, we combine a comprehensive portfolio of medical communications services with expertise in all major therapeutic areas, as well as clinical development, patient engagement, real-world evidence, health economics, market access, regulatory, and more — to communicate vital data to your stakeholders.
Our team consists of Medical Affairs strategists who collaborate with highly skilled PhD, PharmD, and MD writers, seasoned medical editors, meeting logistics experts, and creative, digital, and design specialists. Together, we enhance educational experiences and bring your science to life across every stage of development.

Our writers have deep experience across therapeutic areas, resulting in more relevant, impactful medical communications.
Drug safety & pharmacovigilance
In the rapidly evolving landscape of innovation-driven medicine, ensuring drug safety has become increasingly complex. With vast amounts of data from diverse sources, managing safety information is more challenging than ever. At Parexel, we leverage nearly 40 years of expertise to navigate these complexities, providing comprehensive pharmacovigilance services that prioritize patient safety throughout a product's lifecycle. Our integrated approach combines cutting-edge technology and AI-powered solutions to deliver actionable insights and streamline processes.
With a global team of over 3,000 safety professionals, we support a wide range of therapeutic areas, ensuring regulatory compliance and unwavering commitment to patient health. Explore how our tailored solutions can help you meet the challenges of modern drug safety.
Integrated evidence generation planning
Parexel offers expertise in developing and delivering IEGP for various organizations, from big pharma to small biotech. Our services include early-stage strategy development, tactical planning, function-specific support, and advisory services.
By partnering with us for integrated evidence planning, you can maximize your assets' potential, streamline development processes, and improve commercial outcomes in a competitive market.
Operational excellence
Constantly evolving how we deliver trials
The purpose of our Operational Excellence and Delivery Office is to continuously and consistently improve the way we run your trials. By assembling our most experienced, cross-functional team members, we create best practices business wide that accelerate timelines, generate compelling evidence, promote innovation — and empower us to deliver With Heart™.
Learn more about our strategy to deliver the best outcomes for your clinical development programs.
Related Insights
Article
Beyond traditional outsourcing: Building bespoke drug development partnerships for long-term value
Feb 19, 2026
Blog
New EU pharmaceutical legislation: Strategic implications for drug developers
Feb 17, 2026
Blog
EMA’s updated guideline on pharmaceutical quality of inhalation and nasal products put to a peak flow test
Feb 10, 2026
Blog
Decoding FDA’s new flexible CMC requirements for cell and gene therapies
Jan 26, 2026
Blog
UK's nonclinical roadmap: MHRA joins global regulatory shift toward NAMs with detailed goals and timelines
Jan 8, 2026
Blog
EU Biotech Act: Strategic considerations for biotech companies
Dec 22, 2025
Blog
EMA’s evolving stance on external controls: Key takeaways and preparation strategies for sponsors
Dec 10, 2025
Blog
FDA's new ‘plausible mechanism pathway’: Transforming regulatory approval for personalized gene therapies
Dec 10, 2025
Playbook
Strategic outsourcing redefined: Building partnerships that propel innovation
Dec 5, 2025
Webinar
Global trial expansion: Navigating Australia & LATAM's evolving clinical research landscape
Dec 2, 2025
Blog
Optimize your post-approval CMC change management in China with the ICH Q12 tool
Nov 24, 2025
Blog
New FDA biosimilars guidance: CES waiver pathway aligns with global regulatory approaches
Nov 11, 2025
Video
Oncology experts roundtable: Practical insights on FDA draft guidance discussing OS data in cancer trials
Oct 24, 2025
Video
New Era of JCA in Europe
Oct 17, 2025
Webinar
Accelerating delivery of radiopharmaceutical trials
Oct 3, 2025
Audiogram
New FDA regulatory initiatives improving rare disease drug development
Sep 23, 2025
Blog
Operational strategies for faster, more efficient pivotal NSCLC trials
Sep 17, 2025
Blog
Be ready for unannounced foreign inspections by US FDA
Sep 5, 2025
Blog
Accelerate global drug development: Navigating China's evolving pharmaceutical regulatory landscape
Sep 4, 2025
Audiogram
Fast-track to UK market: Mastering the MHRA's recognition procedure
Sep 3, 2025
Related Insights
Article
Beyond traditional outsourcing: Building bespoke drug development partnerships for long-term value
Feb 19, 2026
Blog
New EU pharmaceutical legislation: Strategic implications for drug developers
Feb 17, 2026
Blog
EMA’s updated guideline on pharmaceutical quality of inhalation and nasal products put to a peak flow test
Feb 10, 2026
Blog
Decoding FDA’s new flexible CMC requirements for cell and gene therapies
Jan 26, 2026
Blog
UK's nonclinical roadmap: MHRA joins global regulatory shift toward NAMs with detailed goals and timelines
Jan 8, 2026
Blog
EU Biotech Act: Strategic considerations for biotech companies
Dec 22, 2025
Blog
EMA’s evolving stance on external controls: Key takeaways and preparation strategies for sponsors
Dec 10, 2025
Blog
FDA's new ‘plausible mechanism pathway’: Transforming regulatory approval for personalized gene therapies
Dec 10, 2025
Playbook
Strategic outsourcing redefined: Building partnerships that propel innovation
Dec 5, 2025
Webinar
Global trial expansion: Navigating Australia & LATAM's evolving clinical research landscape
Dec 2, 2025
Blog
Optimize your post-approval CMC change management in China with the ICH Q12 tool
Nov 24, 2025
Blog
New FDA biosimilars guidance: CES waiver pathway aligns with global regulatory approaches
Nov 11, 2025
Video
Oncology experts roundtable: Practical insights on FDA draft guidance discussing OS data in cancer trials
Oct 24, 2025
Video
New Era of JCA in Europe
Oct 17, 2025
Webinar
Accelerating delivery of radiopharmaceutical trials
Oct 3, 2025
Audiogram
New FDA regulatory initiatives improving rare disease drug development
Sep 23, 2025
Blog
Operational strategies for faster, more efficient pivotal NSCLC trials
Sep 17, 2025
Blog
Be ready for unannounced foreign inspections by US FDA
Sep 5, 2025
Blog
Accelerate global drug development: Navigating China's evolving pharmaceutical regulatory landscape
Sep 4, 2025
Audiogram
Fast-track to UK market: Mastering the MHRA's recognition procedure
Sep 3, 2025



